Sunitinib (Sutent) Experience in Use
 GIST Support International posed questions about the drug sunitinib (Sutent) for treating gastrointestinal stromal tumors (GISTs) to Peter Reichardt, MD PhD. Dr. Reichardt is Assistant Professor and Head of the Department of Hematology and Oncology in the Sarcomacenter at the HELIOS Klinikum Bad Saarow, Germany. Dr. Reichardt trained at the University of Heidelberg and at the MD Anderson Cancer Center. Dr. Reichardt is active in the EORTC Soft Tissue and Bone Sarcoma Group, the EORTC Gastrointestinal Tract Cancer Group, and the Connective Tissue Oncology Society. He has co-authored numerous publications on the management of GIST. He is very active in coordination of clinical trials for GIST. In Germany Dr. Reichardt assists the GIST patient community as chairman of the scientific committee of the GIST patients organization “Das Lebenshaus.†He co-authored a book for GIST patients that you can download from the website of Das Lebenshaus.
GIST Support International posed questions about the drug sunitinib (Sutent) for treating gastrointestinal stromal tumors (GISTs) to Peter Reichardt, MD PhD. Dr. Reichardt is Assistant Professor and Head of the Department of Hematology and Oncology in the Sarcomacenter at the HELIOS Klinikum Bad Saarow, Germany. Dr. Reichardt trained at the University of Heidelberg and at the MD Anderson Cancer Center. Dr. Reichardt is active in the EORTC Soft Tissue and Bone Sarcoma Group, the EORTC Gastrointestinal Tract Cancer Group, and the Connective Tissue Oncology Society. He has co-authored numerous publications on the management of GIST. He is very active in coordination of clinical trials for GIST. In Germany Dr. Reichardt assists the GIST patient community as chairman of the scientific committee of the GIST patients organization “Das Lebenshaus.†He co-authored a book for GIST patients that you can download from the website of Das Lebenshaus.
In June 2008 Dr. Reichardt presented a paper at the ASCO meeting concerning the worldwide "treatment-use trial" of sunitinib for GIST patients. This trial makes sunitinib available to patients in countries where the drug is not yet approved. You may view his complete set of slides at the ASCO website by linking the title below.
Detailed analysis of survival and safety with sunitinib (SU) in a worldwide treatment-use trial of patients with advanced GIST.
Below are Dr. Reichardt’s responses to our questions about sunitinib (Sutent).
1. The 2006 Demetri et al paper on the Phase III trial of sunitinib yielded a median time-to-progression of 27 weeks in the sunitinib arm based on 207 subjects. What time-to-progression was found in the larger world-wide trial? Also, is there any indication which of these or other factors may have influenced the difference?
a. Different initial performance status of subjects (how sick they were)
b. Different proportion of exon-9-mutant subjects and wild-type subjects
c. Different prior duration or dose on imatinib
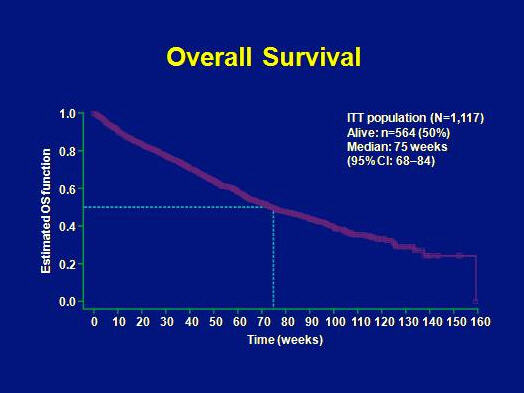
The median time to tumor progression in the treatment-use trial was 41 weeks and the overall survival was 75 weeks. These results compare favourably to the results from the phase III trial.
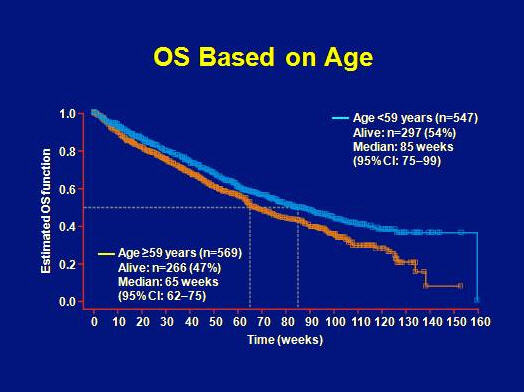
Overall survival was better for patients with age <59 as compared to patients >59 years (85 weeks vs. 65 weeks, respectively).
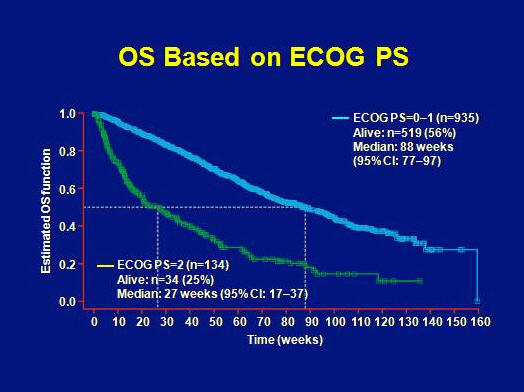
An even larger difference in OS was found relative to ECOG performance status with 88 weeks in case of ECOG 0-1 and only 27 weeks for ECOG 2.
Mutational status of patients in the treatment-use trial is unknown.
2. Because there are many patients who do not derive benefit from sunitinib after progression on Gleevec, it is difficult for patients to judge how effective Sutent can be for those patients who do derive maximum benefit from it. Are there patients who have been stable on Sutent for long durations? If so, what characteristics distinguish these patients from those who discontinue the drug, and how long have such patients remained on Sutent? (mutation status? Intolerance of imatinib? Primary resistance to imatinib?)
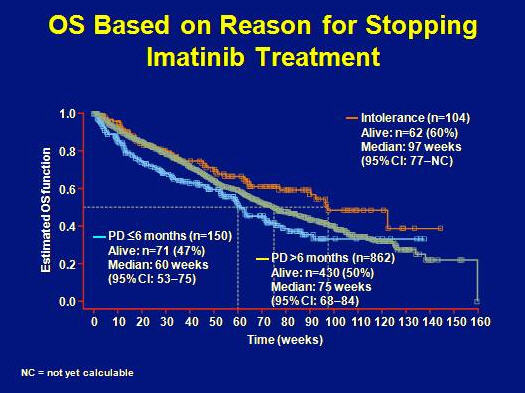
Approximately 20% of patients have been stable for 2 or more years.
OS was longest for patients with age of <59 years, performance status ECOG 0-1, pretreatment with a maximum of 400 mg of imatinib or intolerance to imatinib.
Worst OS was attributed to age above 60 years, poor performance status, pretreatment with higher doses of imatinib and primary resistance to imatinib.
3. The 2006 Demetri et al paper alluded to differences in Sutent response by mutation type, and these data have been reported at ASCO 2006 by Heinrich. Will analyses by mutation type be performed on the continuation trial? What is the current knowledge on this topic?
No mutation analysis data available.
4. Do you recommend mutation testing for patients prior to considering whether to switch from Gleevec to Sutent versus to enter a clinical trial, or should all such patients (even exon 11 patients with secondary exon 17 mutations) try sunitinib after experiencing resistance to Gleevec?
Sunitinib is considered standard of care for second-line therapy (having failed imatinib) irrespective of mutational status.
5. How long a washout period (if any) would you recommend between stopping Gleevec and starting Sutent?
Sunitinib can be started 24 hours after the last imatinib dose (Casali et al. ASCO 2008, abstract 10557).
6. Do the side effects reported for Sutent tend to diminish with the passage of time? Can dose reduction help control side effects while maintaining efficacy?
Side-effects like diarrhea and hand-foot-syndrome in most cases do not diminish over time. Dose reductions are often needed and help control the side-effects. However, some patients are not able to tolerate effective doses of sunitinib. This means that the disease might progress due to the necessary dose reductions.
7. What is currently known about the relative effectiveness and tolerability of daily dosing versus the cycle + washout dosing schedule for sunitinib?
The effectiveness seems to be at least comparable and the tolerability is superior with the continuous dosing schedule. This is likely to become the standard in the future.
8. Are there any data on the blood plasma levels of sunitinib needed for efficacy?
Very limited evidence, but blood levels seem to correlate with efficacy as for imatinib. This is still under evaluation.
9. Is there any clinical experience, or any trial planned, to test sunitinib as first line treatment for Exon 9 and wild-type GIST patients?
No trial planned so far. No data available.
10. There is an ongoing trial to compare results for patients who have progression on 400 mg of imatinib and then either:
A. escalate to 800 mg imatinib, or
B. switch to Sunitinib.
While we await the outcome of this trial, is there any clinical experience with this choice? Are there any types of patients who should consider one option rather than the other?
There is no information available yet. Recruitment is slow.
Trial inclusion is done without testing of mutational status or imatinib blood levels.
Outside of this trial, standard of care is escalation of imatinib.
View a poster about the Sutent worldwide treatment-use trial:
CLICK TITLE BELOW to view an Adobe pdf file of a poster presented at the November 2008 CTOS meeting. If you do not already have the free Adobe Reader software on your computer, you can download it free from the Adobe website. Note that once you open the file, you will need to use the magnification control of the Adobe Reader software to enlarge the image to view one column at a time.
Detailed Analysis of Survival and Safety with Sunitinib in a Worldwide Treatment-use Trial of Patients with Advanced Imatinib-resistant/intolerant Gastrointestinal Stromal Tumor
by B. Seddon, P.
Reichardt, YK Kang, W. Ruka, A Nieto, A Breazna, T. Lechner, and GD Demetri.
Poster presented in London, November 2008, at the meeting of the Connective Tissue Oncology Society.

